價格:免費
更新日期:2020-04-09
檔案大小:7.2 MB
目前版本:1.06
版本需求:系統需求:iOS 12.0 或以後版本。相容裝置:iPhone、iPad、iPod touch。
支援語言:英語
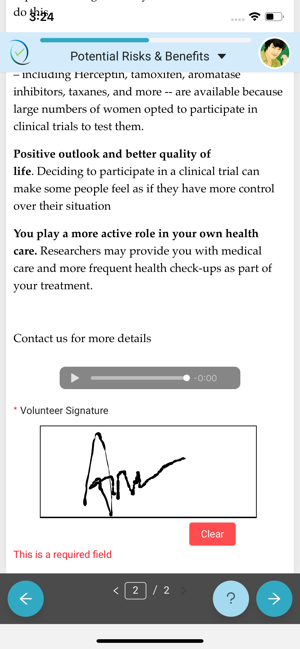
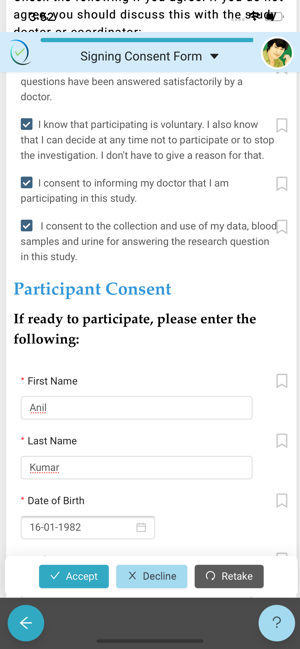
Any volunteer who wishes to consent to take up a Clinical Trial, needs to consent using the ClinConsent App.
ClinConsent removes the risk of mishandled Consent documents and subsequent issues in regulatory inspections. It simplifies the informed consent for sponsors, Site managers, IRB, and Ethics Committees with an easy-to-use technology platform. This dramatically improves the visibility and transparency of the Consent process and leads to improved compliance.
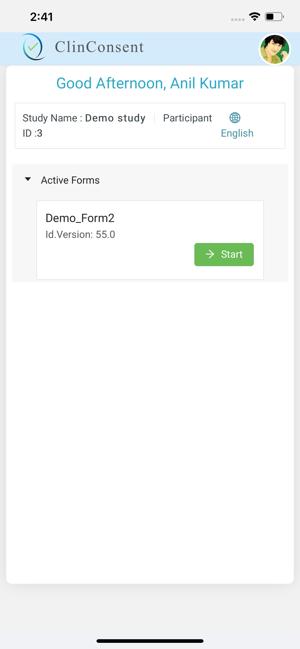
A versatile product that can be used on most devices and across different platforms.
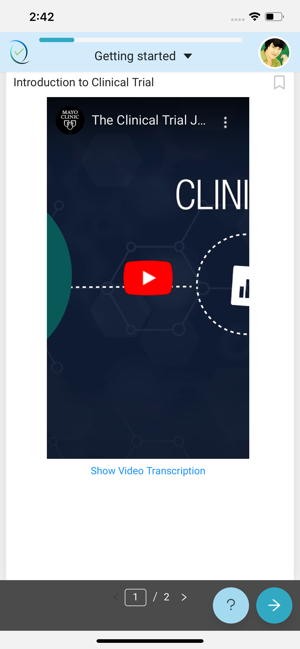
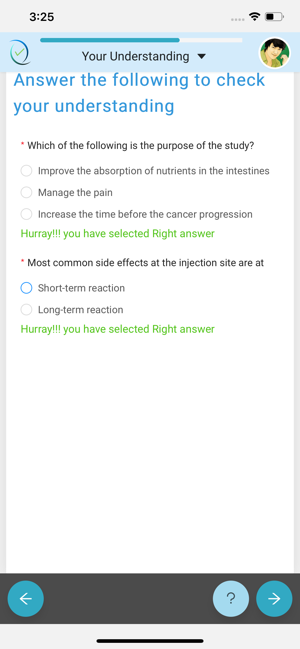
Simple, user-friendly Electronic Consent. Patient-centric design keeps engagement and adherence up, while site staff is freed from time-consuming explanations and able to focus on higher value patient-focused efforts.
Work flow:
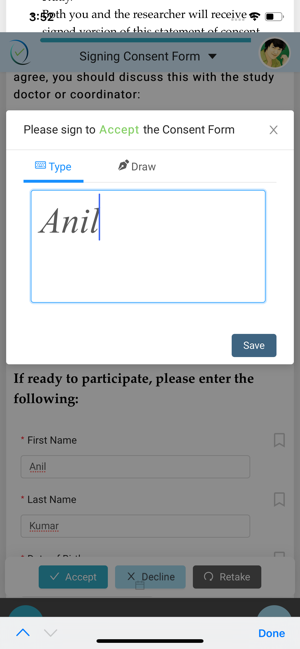
A Clinical Coordinator will register the eligible participants and enter their e-mail address in the ClinConsent portal. Once registered and eConsent is assigned, an e-mail will be sent to the participants with ClinConsent App login credentials to take the consent.

支援平台:iPhone, iPad
