價格:免費
更新日期:2018-06-04
檔案大小:95.1 MB
目前版本:1.4.8
版本需求:需要 iOS 7.0 或以上版本。與 iPhone、iPad 及 iPod touch 相容。
支援語言:德語, 法文, 瑞典文, 義大利文, 英語, 荷蘭文, 葡萄牙文, 西班牙文

IBDoc® offers an easy and intuitive solution to measure and track the inflammation in patients suffering from Inflammatory Bowel Disease (IBD) such as ulcerative colitis and Crohn’s Disease.
As part of the IBDoc® calprotectin home test, CalApp® allows to measure the calprotectin concentration in a stool sample using a smartphone. The app contains an easy to understand tutorial explaining the test procedure step by step from collection of the stool sample to sample extraction and loading of the pregnancy test-like test cassette. Once the test cassette is prepared, the CalApp® controls the mobile device camera to take a picture of the test cassette. The optical signal of the test is analysed with the help of a specific barcode on the test cassette. The signal is translated into a quantitative result that indicates the calprotectin concentration in the stool sample.
The test information is managed on the smartphone itself and at the same time transmitted via a secure and encrypted connection to the IBDoc® Portal. The test results and connected patient information are stored securely on the IBDoc® Portal and can only be accessed by both the patient and his responsible health care professional.
The CalApp® features include:
-tutorial with schematic drawings and text explaining the test procedure step by step

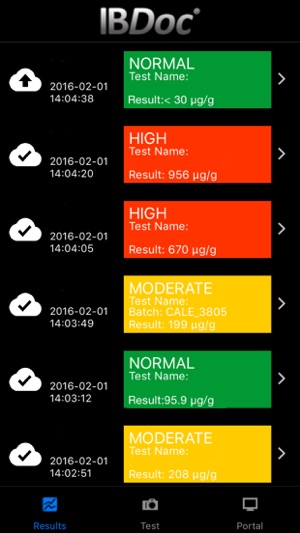
-A list of all test results performed on the device
-help menu with a tutorial video and support contact
---------------------------------------------------
IMPORTANT NOTICE
---------------------------------------------------
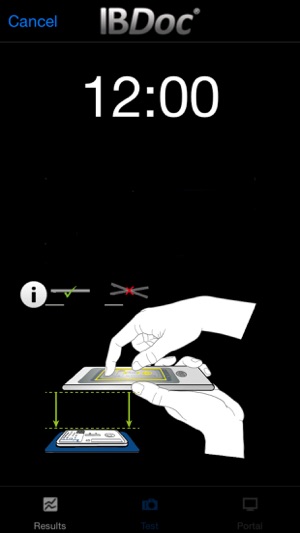
Please be aware that the CalApp® only works with an IBDoc® Account and an IBDoc® test kit. This Account has to be set up by a clinically trained professional. Please contact your IBD doctor or IBD clinic for additional information.
Please read the Instruction for Use included in the IBDoc® test kit carefully before performing the first test.
CalApp® has been validated for specific smartphone models to ensure accurate results, please consult the list of validated smartphones here: www.ibdoc.net/support
A data connection is required. Depending on your carrier additional costs might arise.
--------------------------------------------------

BÜHLMANN Laboratories AG declares that the CalApp® software meets the essential health and safety requirements of EC Directive on in vitro diagnostic medical devices 98/79/EC and the Canadian Medical Devices Regulations SOR/98-282, and is in conformity with the relevant sections of applicable harmonized standards and other normative documents.
CE-marked product: CE0123
IBDoc® and CalApp® are registered trademarks of BUHLMANN Laboratories AG in many countries.
支援平台:iPhone
